Q&A with Paul Nghiem, MD, PhD

The Skin Cancer Foundation spoke with Paul Nghiem, MD, PhD, about landmark progress in the detection, monitoring and treatment of patients with Merkel cell carcinoma (MCC), a rare but dangerous skin cancer. Dr. Nghiem (pronounced NEE-em) is the George F. Odland Endowed Chair and head of Dermatology at the University of Washington in Seattle. He is one of the foremost experts on MCC in the U.S.
Questions and answers
Q: Melanoma kills more people than any other form of skin cancer. But while Merkel cell carcinoma (MCC) is much rarer than melanoma, it is more dangerous case by case. What makes it more dangerous?
A: Early MCCs are typically far less conspicuous than early melanomas. They don’t look concerning, so they often end up being detected at a more advanced stage, when they have metastasized (spread) to other parts of the body and are harder to cure. In contrast, many melanomas are actually found at the in situ stage (stage 0), when they are contained in the very top layer of the skin (epidermis) and have not invaded into the deeper layers. These in situ melanomas are easy to treat and have virtually no chance of metastasizing. But out of more than 1,000 cases of MCC we are following, we have had only two in situ lesions. It is very rare to detect them at such an early time.
When you first find a melanoma or MCC tumor, the initial concern is whether tumor cells may have metastasized from the lesion (the primary tumor) to the nearest lymph nodes. The standard way of checking this today is the sentinel node biopsy (SLNB). In this test, the physician verifies that no cancer cells have spread to the lymph nodes by removing and microscopically examining the first few nearby nodes that receive lymph fluid drainage from the primary tumor. With melanoma, in most cases, the tumor is small and at an early enough stage that you don’t need to do an SLNB since it is very unlikely to have metastasized. However, even the very smallest MCC, as little as four or five millimeters in diameter, has a 15 percent chance of having spread to the lymph nodes or beyond, so we consider doing an SLNB even if an MCC is very small.
Q: What can people do to discover more of these lesions when they’re readily curable? How can they recognize potential MCCs earlier?
A: The classic ABCDE signs of melanoma don’t work for Merkel cell carcinoma. Instead, we have the acronym, AEIOU, which can help with early recognition: Asymptomatic/lack of tenderness; Expanding rapidly; Immune suppression; Older than 50 years; and Ultraviolet-exposed/fair skin. About 63 percent of MCC patients have rapidly expanding lesions, and close to 90 percent have three or more of the AEIOU features. It is important to note, however, that many lesions with several of the AEIOU features do not prove to be MCC—some are benign, and others may be a different skin cancer.
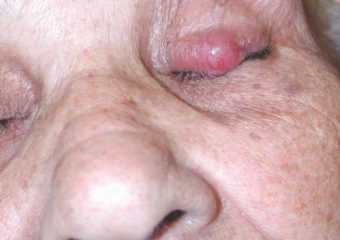
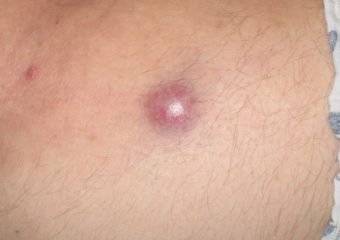
Photos courtesy of Paul Nghiem, MD, PhD
Q: Once an MCC has metastasized to the lymph nodes or beyond, what are the patient’s chances of survival?
A: That’s hard to say right now, because thanks to new treatments, survival is changing quickly for the better. I used to feel hopeless if somebody developed metastatic MCC, but now it is clearly not hopeless. There have been major changes in the field, with a real possibility of cure or near-cure for advanced MCC. I now feel that at least half of the patients with metastatic MCC will be effectively treated.
Q: What treatments have made the difference?
A: In the past, we treated advanced Merkel cell carcinoma with chemotherapy. This approach would shrink tumors in over half of cases, but the benefit would last only three months on average. When the cancer returned, it was resistant to many drugs, and the immune system had been suppressed by the chemotherapy, compounding problems for what is a very immune-sensitive cancer. However, checkpoint inhibitors are an exciting new class of immunotherapies (treatments that boost the ability of the immune system to fight a disease), and have become the standard of care for advanced MCC. By blocking protein receptors that normally keep the immune system in check, these drugs stimulate T cells to fight the tumor.
In March 2017, the US Food and Drug Administration (FDA) approved an immune-stimulating drug, avelumab (Bavencio), for the treatment of adults and pediatric patients 12 years and older with metastatic MCC. FDA approval, the first ever for advanced MCC, was based on data from a clinical trial in which 33 percent of the patients experienced complete or partial shrinkage of their tumors, even though they all previously received chemotherapy for their MCC. Subsequent studies of avelumab have shown higher response rates when given as ‘first line’ therapy (before chemotherapy), with about 50% of patients benefitting.
Injected intravenously, avelumab stimulates T cells by blocking a molecule called PD-L1 (programmed death-ligand 1) that links up with PD-1 to inhibit these critical immune cells.
In 2018, the FDA approved pembrolizumab (Keytruda) for treating MCC, after a study of patients with recurrent locally advanced or metastatic MCC who had not received previous systemic therapy for the disease showed a 56 percent objective response rate (ORR).
In 2023, the FDA granted accelerated approval to retifanlimab-dlwr, also a PD-1 inhibitor, as a treatment for adults with metastatic or recurrent locally advanced MCC. Its safety and efficacy were based on data from the POD1UM-201 trial that evaluated the treatment in adults with metastatic or recurrent locally advanced MCC who had not received previous systemic therapy for the disease. Those patients showed an ORR of 52 percent.
I used to feel hopeless for patients with metastatic MCC. Now, it is clearly not hopeless.
Q: Is there any preferred order in which treatments should be given now? Do you have to administer chemotherapy or radiation first, before using one of the checkpoint blockade therapies?
A: Until recently, chemotherapy was the frontline therapy. But if you treat advanced MCC with a checkpoint inhibitor before doing chemotherapy, about 60 percent of patients respond. If you wait until the patient has failed one round of chemotherapy, it appears that about 40 percent of patients respond. And after two or more rounds of chemotherapy, about 20 percent of patients respond. So, usually the right approach now seems to be to use the immune-stimulating drug first, then decide what to do next based on the response. Some patients will have a complete response, with total remission of the disease. Some patients’ tumors will partially respond, often decreasing by 70 percent in tumor volume, which is still a major response. Many of these patients end up having a low level of disease that does not affect their health at all. One woman I treated recently had a partial response for a year, then it changed to a complete response. But usually, if the drug is going to work, the response comes fast (within 1-2 months), and most responses last well over a year. However, we are uncertain what will happen when the drug is stopped.
Since avelumab is now FDA-approved for MCC that has or has not been previously treated with chemotherapy, it is now being used preferentially as a first-line therapy for metastatic MCC, while chemotherapy is not favored in most cases. Current data suggest that immune therapy works better if given prior to chemotherapy, which tends to suppress the immune system and make tumors more resistant to a variety of treatments.
Although chemotherapy is no longer the usual frontline treatment, radiation may in some cases be used to good advantage early in the game. It can synergize nicely with immunotherapy. The immune system does better at eliminating small tumors than large tumors, and radiation can effectively shrink tumors. If the response to immune therapy is not complete, individual non-responding lesions can sometimes be treated with a little bit of radiation therapy to make the job of the immune system easier.
Q: What about the other FDA-approved checkpoint blockade therapies for melanoma, nivolumab (Opdivo®) and combination ipilimumab (Yervoy®)-nivolumab? Are they being tried for MCC as well?
A: There is an increasing amount of data available on how the ‘ipi-nivo’ combination works in MCC. Its major application has been for patients who do not respond to a single immune-stimulating agent (avelumab or pembrolizumab). For such ‘refractory’ patients, it appears that the ipi-nivo combination can help in about 20 to –30 percent of cases. In contrast, ipi-nivo is not usually used initially because the approximate ~60 percent response rate for pembrolizumab or avelumab alone is quite high,and including ipilimumab (Yervoy) in immune therapy is well known to add significant side effects compared to a single PD-1 pathway drug (such as avelumab or pembrolizumab.).
Q: What happens next if none of the initial checkpoint therapies work?
A: This is a huge area of interest and research. As noted above, the ‘ipi-nivo’ combination can help in about 20 to -30 percent of MCC cases that do not respond to a single immune checkpoint therapy. It is also possible that local injections of immune-stimulating therapies might be combined with a systemic agent such as immunotherapy. Other approaches being explored include giving patients genetically optimized cancer-specific killer T cells, similar to ‘CAR-T’ cells that have proven beneficial for certain blood cancers.
Q: You said you expect about half the patients to do well on the checkpoint blockade therapies. That suggests that about half won’t. Since these are expensive treatments with some potentially serious side effects, is there any way of knowing in advance which patients will benefit, so that you can start the predicted non-responders with a more beneficial treatment?
A: Although more than half of patients respond, for about 40 percent of patients, the cancer continues progressing as if you were giving no treatment at all. We and many others are working very hard to solve that problem, and I am delighted to say that there is progress in this area after more than five years of work. It appears that patients who have good numbers of cancer-specific T cells in their blood are vastly more likely to respond to checkpoint inhibitors than those who do not. Fascinatingly, the number of cancer-specific T cells in the tumors does not predict response. To use a military battle analogy, it appears that the number of effective troops available as backups (in the blood) is critical for winning the war. In contrast, the number we find on the battlefield (in the tumor), is not important. These findings do suggest that if we can increase the number of cancer-specific T cells available to the patient, we may be able to help them better fight the cancer.
Q: How about after a course of treatment has been administered? Is there a way of knowing early on whether the cancer is starting to come back?
A: We have made exciting strides in this area. Our team developed a blood test, which looks for certain Merkel cell polyomavirus antibodies in the blood, so that we can detect any recurrences early, when the tumor will be more effectively treatable (see www.merkelcell.org/sero).
In 2008, Patrick S. Moore, MD and Yuan Chang, MD found that most Merkel cell carcinomas are in part caused by a virus. (Sunlight also often plays a role.) We find this Merkel cell polyomavirus in about 80 percent of MCC tumors. When this virus infects cells, it produces oncoproteins (cancer-promoting proteins) that may cause the cells to grow out of control. We do the blood test at different intervals, starting at the time of diagnosis, and if the number of antibodies to these polyomavirus proteins ever increases, it strongly indicates that the patient is having a recurrence.
We are now using this test routinely, because it is very helpful in managing patients over time. About half of patients do not produce any of these antibodies, and they are actually at about 40 percent higher risk of having their MCC recur than those that make the antibodies, so we know we need to follow them closely with other approaches. For patients who make the antibodies it’s relatively simple: If the cancer does not come back, the antibodies fall quickly, and in most cases they are undetectable by one year after treatment. If MCC comes back, the antibodies go up. It gives us a great chance of discovering recurrences early, when they are extremely treatable: Treating a grape instead of a grapefruit (in terms of cancer size) makes a big difference. And with a patient whose antibodies keep falling, the blood test gives us great confidence that the cancer is not recurring, so we can skip or reduce the use of costly imaging scans.
As of 2022, we are involved in developing another test that detects bits of DNA from the cancer that are floating around in the blood. This is called a ‘circulating tumor DNA’ test. It has been used for cancers including breast and colon. It appears to work particularly well for MCC. A major advantage of this test is that it works even in patients who do not produce antibodies to the Merkel virus. Therefore, virtually all MCC patients should have a blood test that is typically more accurate and sensitive than body scans
Q: After you have treated MCC patients, how do you decide how often to see them and how much risk they still have of the cancer coming back?
A: In April 2022 we recently reported that the overall risk of MCC recurrence is 40 percent; this is a significantly higher rate than the more common skin cancers. In addition, it is clear that a patient’s cancer stage at presentation and other risk factors greatly affect their own risk of MCC recurrence. These factors include immune suppression, male sex and advanced age, all of which increase risk of recurrence. Given that 95 percent of recurrences happen in the first three years after diagnosis, the time since diagnosis is a very important factor that determines a patient’s remaining chance that their MCC will still recur. This in turn is very helpful in determining how aggressively patients should be followed.
We have created a web-based ‘risk calculator’. that takes these various factors into consideration. It is available via www.merkelcell.org/recur.
In our daily practice, this has proven incredibly useful. For example, when patients are seeing you for their one-year follow-up visit, it is now possible to tell them how much “residual” risk of disease they have, as compared to their chance just as surveillance began.
In general, we now believe that we don’t have to monitor patients so closely for as many years as we used to do. MCC doesn’t seem to be like melanoma, which often recurs five or 10 years after diagnosis. With MCC, if it comes back, it is usually within two to three years. So, we now think that intensive follow-up for two or three years is usually appropriate, with a markedly less aggressive schedule up to about year five.
Q: Are any other new forms of treatment making a difference?
A: The checkpoint blockade immunotherapies have been the game-changer, and no other therapies being tested have made that kind of difference yet. However, in combination with checkpoint therapy, we are now using patients’ own T cells to fight the cancer. In some cases, we “reprogram” the patient’s cells to attack the cancer by recognizing the virus. In other cases, we find and expand their own Merkel virus-specific T cells. This can give a boost to the immune system, and combining this therapy with checkpoint blockade therapy may be more effective than either approach alone.
We are also looking into combining some intralesional therapies with the checkpoint blockade therapies. In small studies in MCC, they have often shrunk or eliminated the injected tumors and reduced some other tumors throughout the body. Studies are ongoing, but it seems likely that some such “local” immune stimulators given along with immune checkpoint agents will help produce higher response rates than the checkpoint agents given by themselves.
Published on November 7, 2016. Updated September 21, 2022.
